Direct-to-Patient Models: Watch-Outs

Direct-to-patient (DTP) models are the shiny new object inpharma, and every pharma manufacturer is getting in the game now that we haveTrumpRx in the US. But DTP also pulls manufacturers closer to distribution, retail-like operations, and customer service —which means new risks and liabilities show up fast if the program isn’t designed carefully.
Here’s the punchy risk map, organized by the 4 Ps of marketing – Product, Price, Place, and Promotion.
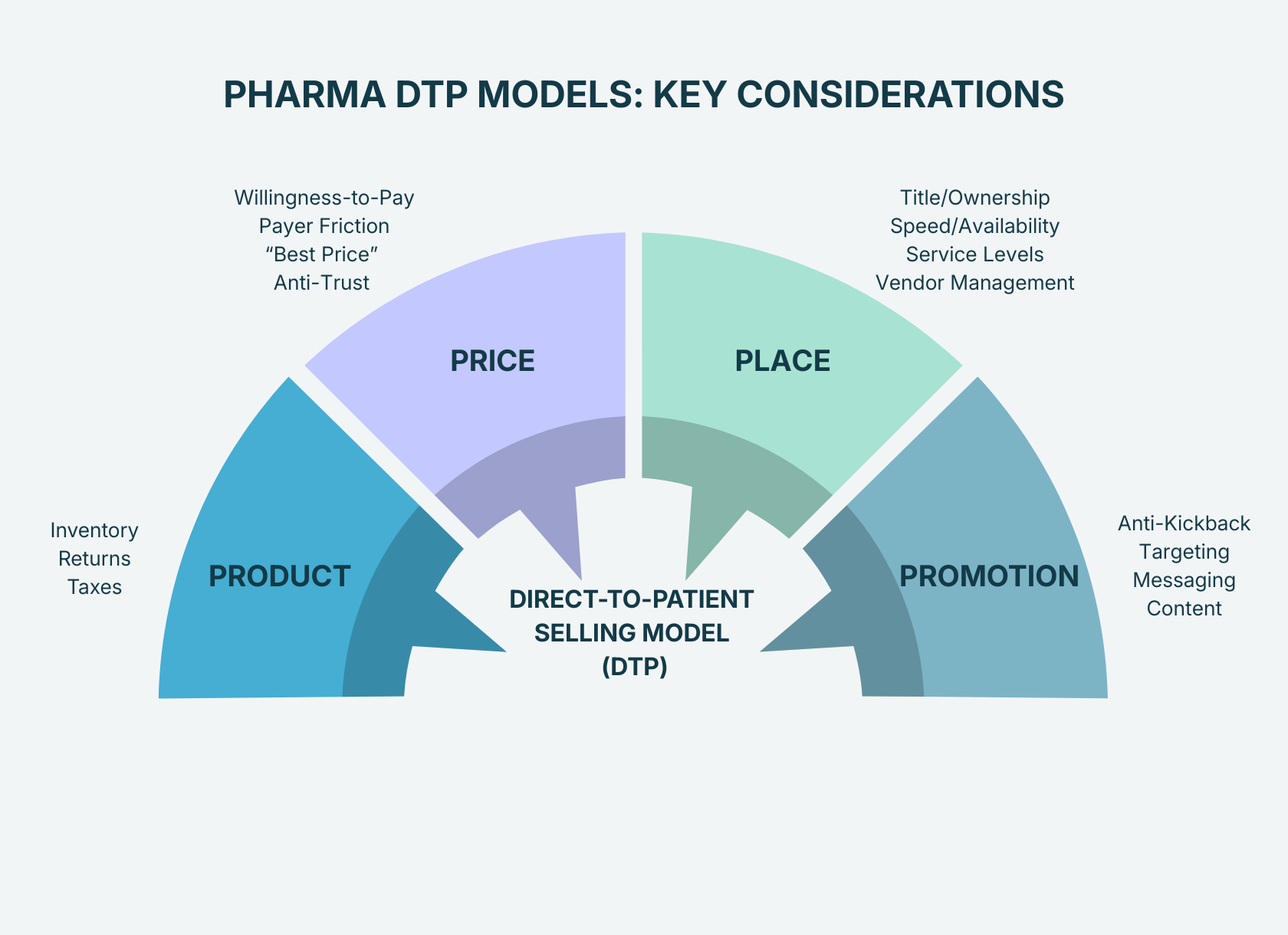
1) Product: Control the chain-of-custody (or it will control you)
Instead of shipping to a small handful of wholesalers & distributors, DTP means you’ll be selling & shipping to thousands of customers, which means you suddenly have the responsibilities of a retailer.
Watch-outs:
- Inventory – No longer selling pallets, you must track sales by the pill bottle, auto-injector, vial, etc. Each unit needs its own serial number, and systems need to be able to account for how many are left to properly plan for outages and restocks. And what happens if there’s a product recall? Will you have enough information to be able to contact each customer about recalls? Are your systems ready to track at this level of detail?
- Returns – What if a patient finds there’s a quality issue and wants to return the product? Will you refund the patient? Does that reset the number of refills available on the prescription?
- Taxes - sales tax needs to be collected for the point-of-destination. For 5 states, sales tax is collected on Rx drugs, so how will you calculate at collect? And how will you fulfill state audit requirements?
2) Pricing: DTP Pricing is visible—and payer conflicts are real
DTP turns pricing into a patient decision-making moment, but it also raises the stakes with payers and government price reporting.
Watch-outs:
- Consumer willingness-to-pay (WTP): If you’re really going direct-to-patient, then you need to pick a price that patients are willing to pay. If you’re just creating a program for namesake only, then your supply chain teams will probably look at you incredulously when you ask them to create new processes to establish chain-of-custody. Do some market research and have proof that people will actually buy.
- Payer contract friction: If your DTP price is significantly below the net price offered to your contracted payers, you will likely risk retaliation the following year. Check your contracts to make sure you’re not violating one of the existing terms either.
- Best price exposure: Government compliance requires that Medicaid & 340b get the “Best Price” on the product, but there are safe harbors when you offer the discount directly to patients. Watch-out for how your legal team defines “directly to patients.” It will impact how you design the whole model.
- Anti-competitive tactic: By setting price transparency well below WAC (wholesale acquisition costs), you may be facing potential non-compliance with US Anti-Trust Laws. Work with your Pricing & Legal teams to make sure you know how to mitigate this risk before you pull any triggers.
3) Place: Pick the right business model
Place is your business model - it’s how patients pay for and receive the product. With all pharmaceutical products, you need a license to dispense so you’re going to need a pharmacy.
Depending on how accessible your product needs to be, you could go with a centralized (single mail-order pharmacy) or a distributed model (multiple pharmacies).
Watch-outs:
- Title/Ownership: Who is taking financial title (aka ownership) of the product? Whoever takes responsibility needs to pay property taxes & insurance. And if title is transferred to the pharmacy, does it still count as a discount offered directly to patients under the Best Price safe harbor?
- Speed/Availability: Can patients wait for their treatment? Or does it need to have same-day accessibility at the nearest CVS?
- Control/Service: The more pharmacies you add, the harder it is to enforce consistent service levels. Supply chain/transportation logistics get tougher too, as you may need product to be sent from one pharmacy to the other in case of outages.
4) Promotion: Support patients compliantly
This is the most sensitive “P” due to the Anti-Kickback Statute. The AKS is meant to prevent financial incentives from improperly influencing medical decisions, and violation against AKS is a criminal offense + risk of exclusion from federal programs (i.e. Medicare).
Watch-outs:
- Inducement optics: If the program becomes “the reason doctors pick your drug,” you’re in risky territory.
- Wrong-audience outreach: Promoting broadly can blur the lines of demand generation vs. patient support.
- Consent + privacy gaps: Enrollment, targeting, and retargeting need tight controls.
The Takeaway
DTP isn’t just “a new channel.” It’s a bundle of product movement, financial flows, and patient engagement that can either feel effortless—or become a compliance and operational headache.
Next Steps
Want to pressure-test your program? We can walk through a 4P risk review and identify where governance, data, or contracts need to be tightened. Speak with one of our experts.
Need help designing the program? We can work with you to design it from scratch. And it won’t just be polished slides, we’ll give you the implementation plans too. Speak with one of our experts.
Patient Support & Solutions
that actually work
Interested in what we can deliver? Get in touch.

.png)
